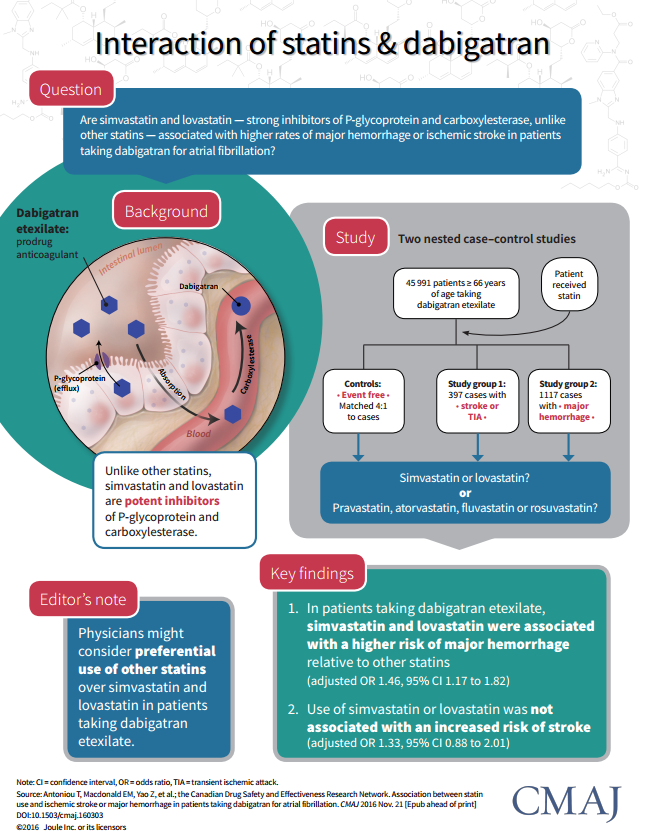
If simvastatin or lovastatin are combined with dabigatran—brand name Pradaxa, an anti-clotting drug—hemorrhage risk increases.
A study published today in the Canadian Medical Association Journal found that within a cohort of almost 46,000 patients treated with dabigatran, the use of simvastatin or lovastatin, relative to other statins, increased the risk of a major hemorrhage by approximately 42 percent.*
Administrative data supported the authors' hypothesis that these two commonly-prescribed, cholesterol-lowering statins would "increase the amount of dabigatran absorbed by the body," reads the St. Michael's Hospital press release, "something other statins would not be expected to do." A higher concentration of dabigatran, in turn, would result in higher bleeding risk.
"Although increased dabigatran absorption might also be expected to improve drug efficacy," write the study authors, "previous studies have found a stronger relation between dabigatran concentrations and major hemorrhage than for ischemic events." In other words, studies have shown a higher concentration of dabigatran does not have much added benefit in terms of preventing stroke, though it does increase bleeding risk.
The specifics of the interaction are interesting, but the study's limitations—using administrative data—make it difficult to speculate about dose-size or dose-timing effects. "We had no data on when dabigatran etexilate was taken in relation to lovastatin or simvastatin," write the authors. "It is possible that separation of these drugs may mitigate the effect of this interaction."
In addition, a relatively small sample of patients taking lovastatin or simvastatin "may have limited our ability to establish whether event risk varies according to statin dose," write the authors. The study was also unable to determine if the drug interaction had any significant effect on stroke risk.
Dabigatran and bleeding: a sore subject
While the study is not a controversial finding in itself, dabigatran—or rather Pradaxa—and bleeding risk are still a sore subject since the drug was criticized for boasting that dose monitoring was unnecessary. "You could have had a much safer drug," said Dr. Deborah Cohen to the New York Times in a controversial 2014 article. Cohen had recently written two high-profile, critical editorials about dabigatran in BMJ.
In response, Doctor John Mandrola corrected "four common mistakes," or rather misstatements made by the New York Times article, on his blog. Significantly, he suggested that Cohen's statement that dose monitoring could have reduced major bleeds by "30% to 40%, compared with well-controlled warfarin," was misleading. "If you made dabigatran perfectly safe (0 bleeds), the best it could have been was 3.36% better than warfarin," Dr. Mandrola wrote. "Dr. Cohen was talking about relative risk reduction, which is not important to the patient considering taking a drug."
Indeed, the 42 percent figure we cited earlier was also a relative risk increase—it could be used misleadingly to sensationalize the risks associated with this drug interaction, when really it's more indicative of the statistical significance of the association.* The authors' conclusion is far from sensational, saying, "Clinicians should consider avoiding simvastatin and lovastatin in older patients receiving dabigatran etexilate who require statin therapy."
* In researching this blog, we found an interesting trail of misinformation and controversy resulting from statistics used in medical journals. I want to clarify that this figure is derived from an approximated and relative risk ratio derived from the study's data, shared as an odds ratio. We'll be following up on this topic—math in medicine—in a later blog.